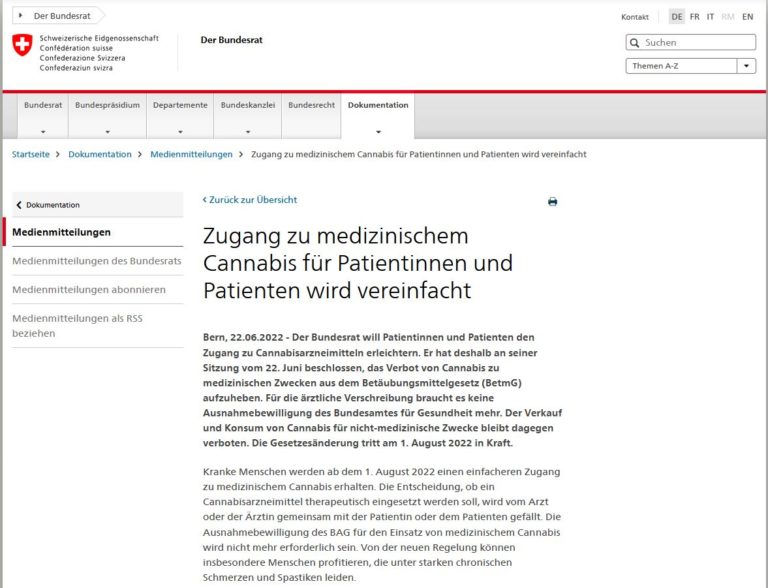
Analysis of the Swiss chemists’ statement saying most CBD cannabis products fail to meet standards. However, it only concerns food and veterinary products.
On February 1st 2022, the Swiss Association of Cantonal Chemists, announced in a press release that, in Switzerland, the vast majority of products sold containing the cannabis extract cannabidiol (CBD) do not meet legal requirements. It was relayed across many media channels, such as swissinfo stating that Most CBD cannabis products fail to meet standards. However, these findings do not apply to the medical/therapeutic cannabis.
- The series of controls conducted in Switzerland concerns food and veterinary products, and not the medical/therapeutic cannabis sold in pharmacies. To note that some pharmacies might, intentionally or by ignorance, sell some products concerned by the study. In pharmacies, customers must request to obtain medical cannabis produced according to medical standards, as defined by swissmedic, the Swiss and European pharmacopeia.
- The current Swiss legal framework limits the availability of cannabinoids to the medical sector (oil and tinctures with any %CBD and %THC), and the smoking sector (CBD flowers <1%THC). CBD is considered a “novel” ingredient or a therapeutic substance, and is therefore not available in food. This positioning is being used by the Swiss Association of Cantonal Chemists to justify the controls.
- Patients should go to pharmacies to get advices regarding CBD and THC. If needed, the cannabis-therapeutics network is available for training Swiss professionals on the use of cannabinoids If you fell that your doctor or pharmacist needs information, please contact us on info@cannabis-therapeutics.ch
The www.cannabis-therapeutics.ch network connects patients, prescribers, pharmacies and producers to make medical / therapeutic cannabis widely available in Switzerland.